Ischemic stroke in MCA dissection with the formation of the double lumen: diagnostic challenges
- Authors: Kalashnikova L.A.1, Filatov A.S.1, Dreval M.V.1
-
Affiliations:
- Russian Center of Neurology and Neurosciences
- Issue: Vol 19, No 2 (2025)
- Pages: 92-96
- Section: Clinical analysis
- Submitted: 24.04.2024
- Accepted: 13.05.2024
- Published: 26.06.2025
- URL: https://annaly-nevrologii.com/pathID/article/view/1125
- DOI: https://doi.org/10.17816/ACEN.1125
- EDN: https://elibrary.ru/YVJJBQ
- ID: 1125
Cite item
Abstract
We describe a patient who experienced a right middle cerebral artery (MCA) stroke at the age of 12. Its clinical manifestations (acute onset of left-sided hemiparesis and headache during swimming) and signs of connective tissue weakness (joint hyperflexibility, increased skin elasticity) suggested right MCA dissection as the stroke cause. However, 1.5T MRI/MRA did not confirm the clinical suspicion: blood flow in the right MCA was preserved, and no intramural hematoma was detected. Only an irregular MCA contour was noted. High-resolution 3T MRI performed seven years later revealed a double lumen in the right MCA — a characteristic dissection sign — confirming the initial clinical hypothesis. This case demonstrates that when MCA dissection is clinically suspected as the cause of ischemic stroke, high-resolution MRI is necessary to verify neuroimaging signs of dissection.
Full Text
Introduction
Spontaneous intracranial artery dissection (ICAD) is an uncommon, understudied, and underdiagnosed cause of ischemic stroke (IS) [1–3]. Compared to extracranial artery dissection, it is less common. ICAD accounts for 12.5–15.8% of dissections in arteries supplying the brain [4], while among all IS cases caused by dissection, the frequency of ICAD is 33.5–48.8% [3]. Among isolated dissections of the anterior, middle (MCA), posterior cerebral, and basilar arteries, the MCA is most frequently affected. Patients with ICAD are younger than those with cervical artery involvement, and ICAD represents one of the causes of IS in children, who rarely experience extracranial artery dissection [1, 5, 6].
Risk factors for dissection include head trauma, physical exertion, straining, recent infection within the month preceding IS, and oral contraceptive use in women [1]. Migraine in the past history is frequently reported by patients with ICAD [3]. The underlying cause of dissection is vascular wall weakness due to dysplasia. Pathomorphological studies reveal dysplastic changes not only in intracranial but also extracranial arteries [7, 8]. Moreover, in patients with dissection, these changes are widespread, including the musculoskeletal system involvement [9].
IS caused by MCA dissection typically manifests acutely with ipsilateral headache. Headache appears simultaneously with focal neurological symptoms, less often it precedes it by several hours or days. Decreased level of consciousness is uncommon and occurs in rapidly progressing dissection leading to MCA occlusion and large cerebral infarction. Transient ischemic attacks preceding IS by several days may be the first sign of developing ICAD [1].
The mechanisms of IS in ICAD include hemodynamic disturbances due to narrowing/occlusion of the arterial lumen by an intramural hematoma (IMH), artery-to-artery embolism from the site of intimal rupture, and block the place оf the M1 MCA segment where the penetrating arteries branch off. Brain infarcts are located in the territory of the perforating branches of the MCA (posterior limb of the internal capsule, head and body of the caudate nucleus, globus pallidus; 45%), less frequently in the cortex and subjacent white matter of the cerebral hemispheres [1, 2].
Radiologic diagnosis of MCA dissection is challenging due to MCA small size and the nonspecificity of many imaging signs. Magnetic resonance angiography (MRA) reveals stenosis or occlusion, sometimes combined with aneurysmal dilation of the MCA. A characteristic feature distinguishing MCA stenosis/occlusion due to ICAD from atherosclerotic plaque or inflammation is the gradual restoration of arterial patency over 1.5–2.0 months in many cases.
The pathognomonic MRI sign of ICAD on vessel wall imaging is the IMH. Its detection, considering the small artery diameter, is facilitated by specialized MRI sequences (T1-weighted fat-suppressed, T1fs) with thin slices on high-field MRI scanners (3T) [1–3, 6, 10, 11]. Seven Tesla MRI scanners have higher resolution, enabling IMH detection not only in the M1 segment but also in the M2 segment [12]. IMH becomes visible 48–72 hours after disease onset. An additional significant feature is the expansion of the artery’s external diameter due to IMH within the vessel wall. A rare neuroimaging sign of MCA dissection is the double lumen, which also requires high-resolution MRI for identification — a technique not universally available, that creates diagnostic challenges. In the literature, we found only one such case description verified by MRA and subtraction angiography.
The limited coverage of ICAD in the literature and the inevitable diagnostic and differential diagnostic difficulties determined the preparation of this publication.
Clinical case report
Patient S., 19 years old, was undergoing examination in the 3rd Neurological Department of the Russian Center of Neurology and Neurosciences from August 23, 2023, to September 1, 2023 with the aim to clarify the cause of IS in the right MCA dated January 19, 2017.
On admission, the patient complained of slight clumsiness in the left hand, pulsating headaches predominantly in the occipital region worsening with movement and accompanied by phonophobia, and dizziness.
Medical history: Since the age of 8 (2012), he has experienced headaches accompanied by phonophobia that intensify with movement. At the age of 10 (2015), brain MRI revealed significant ventricular system dilation. On January 19, 2017, during swimming, he suddenly developed severe short-lasting headache, left-sided limb weakness, and required assistance to exit the pool. He remained conscious but lethargic, provided incorrect parental phone numbers, and had poor recollection of the acute events. He was hospitalized at Morozov Children’s City Clinical Hospital.
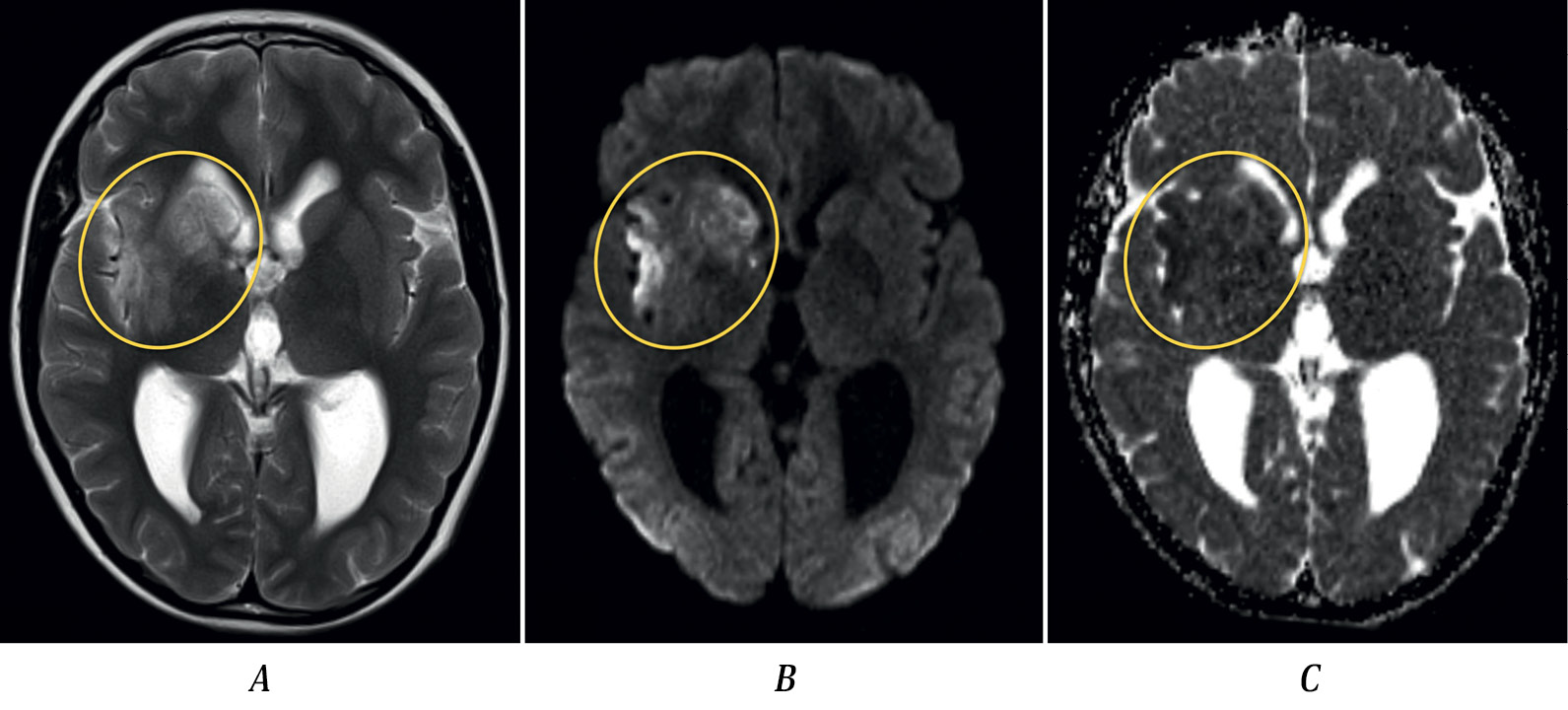
Fig. 1. MRI of patient S. dated 27.01.2017, axial plane.
A — T2-weighted imaging; B — DWI (b-1000); C — ADC map. Heterogeneous areas of increased intensity of MR signal in T2 and DWI (b-1000) sequences, with heterogeneous reduction of MR signal on ADC map (“acute” infarction), are observed in the head of the caudate nucleus, putamen, anterior limb of the internal capsule, and gray/white matter of the insular lobe in the right cerebral hemisphere.
Brain MRI demonstrated an ischemic area in the right MCA territory and hydrocephalus (Fig. 1). MRA performed on January 27, 2017 and analyzed at the Radiology Department of the Russian Center of Neurology and Neurosciences, revealed irregular contours of the M1 segment of the right MCA (Fig. 2, A) and hypoplasia of the A1 segment of the right anterior cerebral artery. No intramural hematoma in the right MCA or pathological vessel wall contrast enhancement was detected. These findings were interpreted as arteriopathy. Echocardiography showed no abnormalities. Routine blood tests, biochemical analysis, and coagulation tests were unremarkable. Antiphospholipid antibodies were negative. Tests for antinuclear factor, anti-DNA, and C-reactive protein were within normal limits. Treatment included heparin followed by acetylsalicylic acid 50 mg and neurotrophic agents. His condition improved, with only mild left arm weakness remaining at discharge. Discharge diagnosis: acute ischemic stroke in the right MCA territory. Arteriopathy.
Following IS, blood pressure began to rise, recently reaching up to 180 mm Hg at times. The patient is currently taking amlodipine and fosinopril. Six months after the ischemic stroke, headaches recurred, now localized in the occipital region rather than the frontotemporal area as before; their character changed from pressing to pulsating and became accompanied by blurred vision, visual field narrowing, and dizziness.
Fig. 2. MRA of patient S., MIP reconstruction, axial plane.
A — 27.01.2017: irregular contours of the M1 segment of the right MCA. MR signal from the A1 segment of the right anterior cerebral artery appears fragmented (hypoplasia?); B, C — 23.06.2023: restoration of normal contour configuration of the right MCA, with visualization of a double lumen in the M1 segment (arrows).
At the age of 15 (2019), the patient was evaluated at the Neurosurgical Department of the Morozov Children’s City Clinical Hospital. A lumbar puncture was performed, and CSF analysis showed protein of 0.416 g/L and no cytosis. In 2022 (at the age of 18), a follow-up MRA (1.5 T) revealed reduced blood flow signal in the right MCA (when viewing an MRI at the RCNN - double lumen in the right MCA). Ventricular enlargement of the brain was observed. On June 23, 2023, high-resolution MRA (3 T) performed at the Russian Center of Neurology and Neurosciences demonstrated a double lumen of the MCA — a sign of arterial dissection (Fig. 2, B, C). On examination general condition was satisfactory. Joint hypermobility (Fig. 3) and increased skin extensibility were noted. Neurological examination revealed slowed movement speed in the left hand. No sensory or coordination deficits were detected. The patient studies at university.
Fig. 3. Hypermobility of the wrist joint.
Discussion
This paper presents a case of a patient who had an ischemic stroke at the age of 12 due to ICAD of the right MCA. The diagnosis was confirmed only 7 years later using high-resolution MRI, which revealed a double lumen in the right MCA — a pathognomonic neuroimaging sign of dissection. The limited understanding and diagnostic challenges of ICAD are also highlighted in the literature [2, 3]. ICAD most commonly leads to intramural hematomas, which narrow or occlude the arterial lumen, thereby causing IS. In such cases, MCA occlusion is often misattributed to thrombosis. Accurate diagnosis is facilitated by brain MRI with T1-weighted imaging with fat suppression (T1fs) using thin slices, given the small diameter of the MCA. IMH in this imaging modality is detectable from 5–7 days of stroke and persists for 1.5–2.0 months. During this period, IMH gradually diminishes with restored arterial patency, though occlusion may persist in some cases.
In our patient despite clinical manifestations characteristic of MCA dissection, T1-weighted fat-saturated MRI did not detect an IMH. The reason was that the IMG did not form, since the blood penetrated the arterial wall through the intimal rupture and made a new lumen in it, which connected distally with the main lumen as a result of secondary intimal rupture. Both lumens were functionally significant, resulting in the absence of hematoma within the vessel wall. The imaging study at acute stroke stage was performed using 1.5 T MRI, which could not visualize the double lumen. In such cases, a comprehensive analysis of clinical and instrumental data helps clarify the cause of IS and determine indications for high-resolution MRI. The IS during swimming involving head rotations and physical exertion, along with the simultaneous onset of headache and focal neurological symptoms, is pathognomonic for ICAD [1]. Additionally, the patient exhibited signs of connective tissue dysplasia (joint hypermobility, increased skin elasticity), commonly found in patients with dissections of brain-supplying arteries [9]. According to morphological studies, connective tissue dysplasia underlies the vascular wall weakness leading to dissection [7, 8]. These features allowed clinical suspicion of dissection, which was not confirmed by neuroimaging during the acute phase and subsequently justified by high-resolution MRI that revealed a double lumen in the MCA.
Differential diagnosis of MCA dissection as a cause of ischemic stroke was carried out with cardiogenic embolism, taking into account the acute development of ischemic stroke. The absence of cardiac pathology on echocardiography, as well as the deep (rather than superficial) location of the cerebral infarction — a typical feature of cardioembolism — ruled out this etiology of IS. Thrombophilia, one of the causes of IS in childhood and adolescence, was excluded due to the absence of systemic venous or arterial thromboses and laboratory markers of thrombophilia.
Treatment of IS caused by ICAD in the acute phase includes antithrombotic therapy to prevent thrombotic complications at the site of intimal rupture. Comparative studies of antiplatelets versus anticoagulants in ICAD have not been conducted. Due to the thin MCA arterial wall, which may predispose to aneurysm formation, high-dose anticoagulants are not safe. In the development of extensive cerebral infarction due to intracranial dissection, the use of anticoagulants is contraindicated due to the risk of increasing IMG. Antiplatelet therapy is preferred in such situation [14, 15].
In conclusion, the diagnosis of MCA dissection relies on a comprehensive evaluation of clinical and instrumental data. Negative MRI findings for IMH in patients with typical clinical manifestations of MCA dissection warrant high-resolution MRI to exclude a double lumen — another pathognomonic neuroimaging feature of ICAD.
About the authors
Ludmila A. Kalashnikova
Russian Center of Neurology and Neurosciences
Author for correspondence.
Email: kalashnikovancn@yandex.ru
ORCID iD: 0000-0003-1142-0548
Dr. Sci. (Med.), professor, principal researcher, 3rd Neurological department, Institute of Clinical and Preventive Neurology
Russian Federation, 80, Volokolamskoye shosse, Moscow, 125367Alexey S. Filatov
Russian Center of Neurology and Neurosciences
Email: kalashnikovancn@yandex.ru
ORCID iD: 0000-0002-5706-6997
Cand. Sci. (Med.), junior researcher, Radiology department, Institute of Clinical and Preventive Neurology
Russian Federation, 80, Volokolamskoye shosse, Moscow, 125367Marina V. Dreval
Russian Center of Neurology and Neurosciences
Email: kalashnikovancn@yandex.ru
ORCID iD: 0000-0002-7554-9052
Cand. Sci. (Med.), researcher, Radiology department, Institute of Clinical and Preventive Neurology
Russian Federation, 80, Volokolamskoye shosse, Moscow, 125367References
- Калашникова Л.А., Добрынина Л.А. Диссекция артерий головного мозга. Ишемический инсульт и другие клинические проявления. М.; 2013. Kalashnikova L.A., Dobrynina L.A. Dissection of cerebral arteries. Ischemic stroke and other clinical manifestations. Moscow; 2013. (In Russ.)
- Debette S, Compter A, Labeyrie M-A., et al., Epidemiology, pathophysiology, diagnosis, and management of intracranial artery dissection. Lancet Neurol. 2015;14(6):640–654. doi: 10.1016/S1474-4422(15)00009-5
- Montalvan V, Ulrich A, Wahlster S, Galindo D. Arterial dissection as a cause of intracranial stenosis: a narrative review. Clin Neurol Neurosurg. 2020;190:105653. doi: 10.1016/j.clineuro.2019.105653
- Chen H, Hong H, Xing S, et al. Intracranial versus extracranial artery dissection cases presenting with ischemic stroke. J Stroke Cerebrovasc. Dis. 2015;24(4):852–859. doi: 10.1016/j.jstrokecerebrovasdis.2014.12.008
- Калашникова Л.А., Добрынина Л.А., Коновалов Р.Н., Кротенкова М.В. Ишемический инсульт у детей при спонтанной диссекции (расслоении) интракраниальных артерий. Вопросы современной педиатрии. 2009;8(1):142–146. Kalashnikova L.A., Dobrynina L.A., Konovalov R.N., Krotenkova M.V. Ischemic stroke in children with spontaneous dissection of intracranial arteries. Current Pediatrics. 2009;8(1):142–146.
- Калашникова Л.А., Древаль М.В., Добрынина Л.А., Кротенкова М.В. Диссекция средней и передней мозговых артерий как причина ишемического инсульта у мальчика 7 лет. Журнал неврологии и психиатрии им. С.С. Корсакова. Спецвыпуски. 2016;116(4-2):89–94. Kalashnikova LA, Dreval’ MV, Dobrynina LA, Krotenkova MV. Middle and anterior cerebral arteries dissection as a cause of ischemic stroke in a 7-year-old boy. S.S. Korsakov Journal of Neurology and Psychiatry. 2016;116(4-2):89–94. doi: 10.17116/jnevro20161163289-94
- Калашникова Л.А., Гулевская Т.С., Ануфриев П.Л. и др. Ишемический инсульт в молодом возрасте, обусловленный стенозирующим расслоением (диссекцией) интракраниального отдела внутренней сонной артерии и ее ветвей (клинико-морфологическое наблюдение). Анналы клинической и экспериментальной неврологии. 2017;3(1):18–24. Kalashnikova LA, Gulevskaya TS, Anufriev PL, et al. Ischemic stroke in young age due to dissection of intracranial carotid artery and its branches (clinical and morphological study). Annals of Clinical and Experimental Neurology. 2017;3(1):18–24. doi: 10.17816/psaic383.
- Калашникова Л.А., Чайковская Р.П., Гулевская Т.С. и др. Разрыв интимы при дисплазии стенки средней мозговой артерии, осложнившийся тромбозом и развитием тяжелого ишемического инсульта. Журнал неврологии и психиатрии им. С.С. Корсакова. Спецвыпуски. 2018;118(3-2):9–14. Kalashnikova LA, Chaĭkovskaia RP, Gulevskaia TS, et al. Intimal rupture of the displastic middle cerebral artery wall complicated by thrombosis and fatal ischemic stroke. S.S. Korsakov Journal of Neurology and Psychiatry. 2018;118(3-2):9–14. doi: 10.17116/jnevro2018118329-14
- Губанова М.В., Калашникова Л.А., Добрынина Л.А. и др. Маркеры дисплазии соединительной ткани при диссекции магистральных артерий головы и провоцирующие факторы диссекции. Анналы клинической и экспериментальной неврологии. 2017;11(4):19–28. Gubanova MV, Kalashnikova LA, Dobrynina LA, et al. Markers of connective tissue dysplasia in cervical artery dissection and its predisposing factors. Annals of Clinical and Experimental Neurology. 2017;11(4):19–28. doi: 10.18454/ACEN.2017.4.2
- Choi YJ, Jung SC, Lee DH. Vessel wall imaging of the intracranial and cervical carotid arteries. J Stroke. 2015;17(3):238–255. doi: 10.5853/jos.2015.17.3.238
- Liu YC, Chung CP, Yip PK, Wang V. Spontaneous middle cerebral arterial dissection presented with limb shaking. Acta Neurol Taiwan. 2009;18(1):26–29.
- Xie X, Zhang Z, Kong Q, et al. M2 middle cerebral artery dissection on 7T MRI. Stroke Vasc Neurol. 2022;7(6):550. doi: 10.1136/svn-2022-001557
- Simsek E, Yilmaz S, Oran I, et al. A rare cause of ischemic stroke in childhood: spontaneous long segment intracranial dissection. Childs Nerv Syst. 2020;36(11):2871–2875. doi: 10.1007/s00381-020-04530-9
- Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery [published correction appears in Vasc Med. 2011;16(4):317]. Vasc Med. 2011;16(1):35–77. doi: 10.1177/1358863X11399328
- Engelter ST, Traenka C, Von Hessling A, Lyrer PA. Diagnosis and treatment of cervical artery dissection. Neurol Clin. 2015;33(2):421–441. doi: 10.1016/j.ncl.2014.12.002